Abstract
Introduction: CPX-351 is a liposomal encapsulation of cytarabine and daunorubicin maintained at a synergistic 5:1 molar ratio. It is approved for the treatment of patients with newly diagnosed therapy related acute myeloid leukemia (t-AML) or AML with myelodysplasia-related changes (AML-MRC). Improved outcomes with CPX-351 vs 7+3 have been reported irrespective of AML subtype (t-AML, AML-MRC), age subgroup (60 to 69 vs 70 to 75 years), prior hypomethylating agent (HMA) exposure and whether the patients undergo transplant. The 2017 European Leukemia Net (ELN) classification stratifies AML into 3 risk groups based on genetic and mutational profile. Our aim was to compare outcomes of patients with AML treated with CPX-351 based on the 2017 ELN risk stratification and also compare outcomes post allogeneic stem cell transplantation (alloSCT) by ELN risk groups.
Methods: We identified all AML patients who received CPX-351 as first line therapy at Moffitt Cancer Center between August 2017 and July 2020. A retrospective review of patient charts was conducted to collect demographics, disease characteristics and outcomes. Overall survival (OS) was calculated for the entire cohort from date of diagnosis. Relapse free survival (RFS) was calculated in those who achieved CR/CRi from the time of remission to the date of relapse or death from any cause.
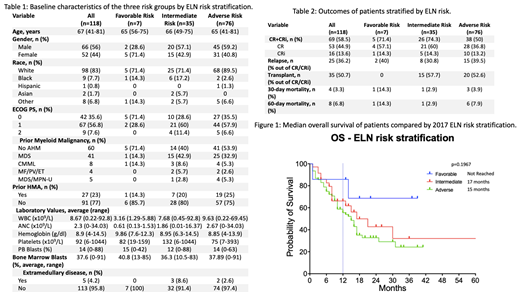
Results: A total of 118 patients with previously untreated AML who received first line CPX-351 were identified. Median age at diagnosis was 67 years (range, 41-81 years). 56% (n=66) patients were male. Based on the 2017 ELN risk stratification the study population was divided into 3 groups: (A) favorable risk (n=7), (B) intermediate risk (n=35), (C) adverse risk (n=76). The favorable risk patients included patients with NPM1 mutation (n=6) and one patient with t(16;16) but also had BM dysplasia.
The CR/CRi rate for the entire study population was 58.5% (n=69). The CR/CRi rate for the favorable and intermediate risk group was significantly better compared to the adverse risk group (71.4% and 74.3% vs 50%, respectively, p=0.0421). The rate of relapse was not statistically different for the 3 risk groups (A- 40%, B- 30.8% and C-39.5%, p=0.76). 30-day mortality was 3.3% for the entire cohort. Of the 26 intermediate risk patients who achieved CR/CRi, 57.7% (n=15) proceeded to an alloSCT in first remission. OS was significantly longer in the transplanted patients than those who did not undergo transplant in CR1 (not reached vs 13 months, p=0.016). Only 1 (6.7%) of the 15 transplant patients relapsed while 7 (63.6%) of the non-transplant patients relapsed (RFS; not reached vs 5 months; p<0.0001).
Of the 38 adverse risk patients who achieved CR/CRi, 52.6% (n=20) underwent allo transplant in CR1. No significant difference was noted in OS between the transplanted patients and the non-transplanted patients. (not reached vs 8 months; p=0.2679). However, transplant in CR1 resulted in a lower rate of relapse (15% vs 67% without transplant; p=0.0023) and improved RFS compared to those adverse risk patients who did not proceed to transplant (not reached vs 8 months; p=0.0007).
At a median follow up of 22 months for the entire cohort, the median OS for the favorable, intermediate and adverse risk groups was not reached, 17 and 15 months, respectively (p=0.1967). At a median follow up of 13 months for patients who achieved CR/CRi, the RFS for the favorable and intermediate groups was not reached and for the adverse risk group was 26 months which lacks statistical significance (p=0.97).
Conclusion: In patients who receive first line CPX-351 for previously untreated AML, ELN risk stratification is able to predict for CR/CRi rates but does not significantly impact OS. In the intermediate risk patients, allo transplant after a CPX-351-induced CR/CRi resulted in significantly improved RFS and OS suggesting that transplant should be considered in ELN intermediate risk patients in first CR after CPX-351.
Sallman: Shattuck Labs: Membership on an entity's Board of Directors or advisory committees; Agios: Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy; AbbVie: Membership on an entity's Board of Directors or advisory committees; Aprea: Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol-Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Syndax: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Incyte: Speakers Bureau; Intellia: Membership on an entity's Board of Directors or advisory committees; Magenta: Consultancy; Kite: Membership on an entity's Board of Directors or advisory committees. Komrokji: AbbVie: Consultancy; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Acceleron: Consultancy; Jazz: Consultancy, Speakers Bureau; Taiho Oncology: Membership on an entity's Board of Directors or advisory committees; PharmaEssentia: Membership on an entity's Board of Directors or advisory committees; BMSCelgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Geron: Consultancy. Kuykendall: Abbvie: Honoraria; BluePrint Medicines: Honoraria, Speakers Bureau; Celgene/BMS: Honoraria, Speakers Bureau; CTI Biopharma: Honoraria; Incyte: Consultancy; Novartis: Honoraria, Speakers Bureau; PharmaEssentia: Honoraria; Prelude: Research Funding; Protagonist: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Padron: BMS: Research Funding; Incyte: Research Funding; Kura: Research Funding; Stemline: Honoraria; Blueprint: Honoraria; Taiho: Honoraria. Lancet: AbbVie: Consultancy; Daiichi Sankyo: Consultancy; ElevateBio Management: Consultancy; BerGenBio: Consultancy; Celgene/BMS: Consultancy; Millenium Pharma/Takeda: Consultancy; Agios: Consultancy; Astellas: Consultancy; Jazz: Consultancy. Sweet: Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees; AROG: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; Bristol Meyers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal